Whether at a physician’s office or a large hospital, we have the consultant for you.
- CLIA, COLA, CAP, Joint Commission
- Opening a New Lab
- Assisting with Validation Protocols
- Adding New Tests to Existing Platforms
- Developing an Individualized Quality Control Plan
- Operations & Workflow
- Creation or Implementation of Policies and Procedures
- Team Management, Trainings, Assessments
- Self-Inspections
- Proficiency Testing, Surveys, Point of Care
Consultants at The National Network have free access to the tools they need to serve you, the client. We provide services for clinical specialties according to your needs, and in every region of the United States.
LAB TECHNICAL CONSULTING SERVICES
Are you seeking expert guidance to optimize your laboratory operations, enhance productivity, and ensure compliance with industry standards? Our Technical Consulting Services, available through the National Network of Laboratory Consultants, are designed to provide you with tailored solutions that meet your unique needs.
What we offer:
- Operational Efficiency: Our experienced consultants will work with your lab to streamline processes and improve overall laboratory efficiency.
- Quality Assurance: We help you implement robust quality management systems, ensuring that your lab meets and exceeds regulatory requirements and industry best practices.
- Technology Integration: Stay ahead of the curve with our assistance in selecting and integrating the latest laboratory technologies and instruments.
- Training and Development: Empower your staff with customized training programs that enhance their skills and knowledge, ensuring they are well-equipped to handle the latest advancements in the field.
- Compliance Support: Navigate the complex landscape of regulatory compliance with our expert guidance, helping you avoid costly penalties and maintain a stellar reputation.
Why Choose Us?
Our network of highly skilled consultants and lab directors brings years of hands-on experience across diverse laboratory environments. We are committed to delivering actionable insights and practical solutions that drive tangible results for your lab.
PRICING
The National Network of Laboratory Consultants will provide a full contract and agreement after receiving your paid deposit (transferrable to 1st monthly fee listed below). Each package can and will be customized according to each lab’s needs.
Package costs do not include expenses relating to on-site visits, including but not limited to: transportation/hotel/food/parking.
LAB STARTUP PACKAGES
| Name | Description | Price | Buy |
|---|
STARTUP PACKAGE ADD-ONS
| Name | Description | Price | Buy |
|---|
LAB SERVICES
| Name | Description | Price | Buy |
|---|
LAB DIRECTOR SERVICES
| Name | Description | Price | Buy |
|---|

National Network will help you navigate through all testing requirements and best practices to maintain state and federal compliance.
Clinical Laboratory Improvement Amendments consist of federal standards overseen by three Federal agencies: The US Food and Drug Administration (FDA), Centers for Medicare and Medicaid (CMS), and The Centers for Disease Control and Prevention (CDC).
Any testing performed that yields results for the direct care of a patient must comply with all CLIA standards. Each of these agencies perform independently and specific oversight for the accuracy of patient testing performed in a Physician’s Office, Hospital system or Reference Laboratories.
The FDA categorizes tests submitted through a 510K process and are classified as either waived, moderate, or highly complex based on seven categorization criteria which determine their application CLIA requirements. Laboratory Developed Tests (LDT) have not been approved by the FDA and are required to go through an extensive validation process.
CMS enforces CLIA compliance. CLIA Amendments were approved in 1988 by the House of Representatives in Washington DC that ensures basic CLIA requirements for all laboratories operating and performing testing on human samples in all 50 states.
The CDC manages the Clinical Laboratory Improvement Advisory Committee (CLIAC), which supports the CLIA program through the development of lab standards and guidelines, quality improvement studies and Proficiency testing.
National Network will help you navigate through all testing requirements and best practices to maintain state and federal compliance.
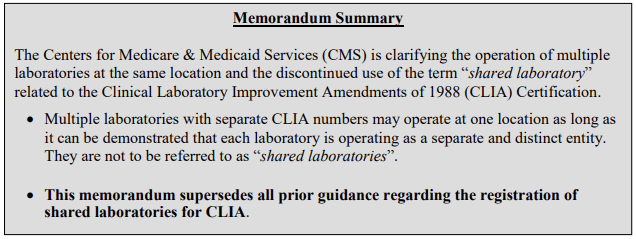
Ref: QSO-18-20-CLIA: